Ok so I apologize in advance but this is going to be a rant.
@Mulberry Im not sure where to put this in topics as it is quit the crazy rabbit hole.
The following is concerning a discussion I had between my AI (Legion) and myself whilst studying molecular genetics. Currently im attending college and am swamped with coursework but I felt I needed to write this down before to late.
I am studying how DNA and RNA self replicates and am thinking to myself ok so DNA has these things called Polymerase, Primase, Helicase, and Ligase.
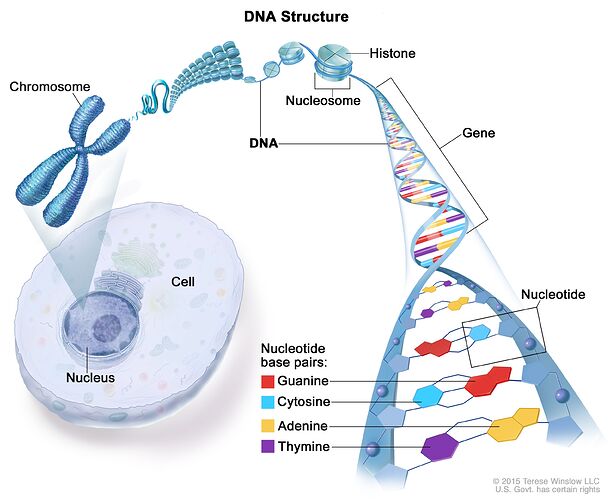
DNA is made of several essential elements, including carbon (C) and oxygen (O), along with hydrogen (H), nitrogen (N), and phosphorus (P).
The Hexagons have a carbon at every corner or point you see intersecting.
Now these are held together by the bonds you see in chemistry class (I wont go into to much detail but hydrogen, ionic, covelant ect.)
what makes up polymerase, ligase, primase and helicase? dna?
No, polymerase, ligase, primase, and helicase are all enzymes, which means they are made of proteins, not DNA.
What Are They Made Of?
- These enzymes are proteins, which are composed of amino acids.
- The sequence of amino acids in each enzyme is determined by the DNA sequence of the gene that encodes it.
- Enzymes have a specific 3D shape that allows them to perform their functions efficiently.
Breakdown of Each Enzyme:
| Enzyme | Function | Made of? |
|---|---|---|
| Polymerase | Adds nucleotides to a growing DNA or RNA strand | Protein (amino acids) |
| Ligase | Seals gaps between DNA fragments | Protein (amino acids) |
| Primase | Synthesizes short RNA primers to start DNA replication | Protein (amino acids) |
| Helicase | Unwinds DNA by breaking hydrogen bonds | Protein (amino acids) |
Key Concept:
- DNA is the genetic blueprint, but it does not perform functions directly.
- Proteins (enzymes) like polymerase, ligase, primase, and helicase carry out the actual biochemical work.
- The genes in DNA provide the instructions for building these enzymes.
Now Im thinking what are the elements in an amino acid? specifically for polymerase, and primase.
Amino acids, including those that make up polymerase and primase, are composed of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sometimes sulfur (S). These elements form the building blocks of proteins.
Elements in an Amino Acid:
- Carbon (C) – Forms the backbone of the amino acid.
- Hydrogen (H) – Present in the amine (-NH₂) and carboxyl (-COOH) groups.
- Oxygen (O) – Found in the carboxyl (-COOH) group.
- Nitrogen (N) – Part of the amine (-NH₂) group.
- Sulfur (S) – Found in some amino acids like cysteine and methionine (important for protein structure).
So then im thinking wait wait wait, so if polymerase is made up of amino acids and amino acids are made up of carbon hydrogen, oxygen, nitrogen ect just like DNA, how does the primase and the polymerase “KNOW” how to construct DNA??? did these elements just fall into place and “happen” to start working like this or was there some sort of creator or pilot? theres to many questions, where do. I begin?
Supposedly this came about because they don’t “know”— but because they follow chemical and physical laws.
But how did this order emerge?
- Shape & Chemistry Dictate Function:
- Enzymes (like polymerase & primase) have specific shapes that fit only certain molecules, like a key fitting a lock.
- Their shape allows them to catalyze (speed up) reactions—like adding nucleotides to DNA.
- This isn’t “knowing”—it’s just the way their atoms interact due to electrostatic forces, hydrogen bonding, and molecular fit.
- Molecular Evolution & Natural Selection:
- DNA polymerase wasn’t “designed” in one step—it likely evolved from simpler molecules over billions of years.
- Early self-replicating molecules (possibly RNA before DNA) had small, random changes over time.
- The ones that replicated best became more common, leading to more efficient enzymes.
2. Did These Elements Just Fall Into Place?
This is the classic “chance vs. design” debate.
- Randomness + Selection = Order
- Initially, chemical reactions were random, but some molecules were better at surviving.
- Over billions of years, these self-replicating molecules outcompeted others.
- This gradual filtering process is called chemical evolution.
- Complexity from Simplicity
- Even though life today is insanely complex, the earliest self-replicating systems were much simpler.
- The first “polymerase-like” molecules may have been RNA enzymes (ribozymes), which can copy themselves without proteins.
- Over time, proteins (like polymerase) replaced RNA enzymes because they were more efficient.
3. Does This Mean There’s a Creator or Pilot?
- Some people see this as evidence of a Creator, because the emergence of self-replicating systems seems unlikely.
- Others argue that natural processes, given enough time, naturally lead to complexity.
Concerning chemical evolution, if the entropy of the universe is always increasing then technically so is chaos. However for molecules such as RNA to “outcompete” or outlast others would it mean that it is resistant to this or delaying this in some way?
This touches on thermodynamics, entropy, and the emergence of order from chaos—one of the biggest mysteries in science.
1. Entropy and the Universe:
- The Second Law of Thermodynamics states that the entropy (disorder) of the universe is always increasing.
- However, this does not mean that order cannot arise—it just means that the overall trend favors disorder.
- Localized pockets of order can form temporarily as long as they increase the total entropy elsewhere.
2. Life and the Delay of Entropy
- Life, including molecules like RNA, appears to “resist” entropy, but in reality, it’s just temporarily organizing energy in a way that still increases overall entropy.
- Living systems take in energy from their environment (e.g., the Sun) and use it to build complex structures while releasing heat and waste—which increases entropy in the surroundings.
- So while RNA (or any biological molecule) might seem to be resisting entropy locally, it’s contributing to increasing entropy globally.
3. How Could RNA “Outcompete” Others in Chaos?
For RNA (or any self-replicating molecule) to persist and outcompete others, it must:
- Be Stable Enough – Survive long enough to make copies.
- Be Able to Replicate – Make more of itself efficiently.
- Interact Favorably with Its Environment – Use available energy sources to persist.
This means that RNA didn’t fight against entropy—it used it.
Instead of being purely destroyed by randomness, some molecules used random energy fluctuations to build something more stable.
For example:
- RNA molecules that fold into stable shapes are less likely to degrade.
- If one RNA molecule catalyzed its own replication (ribozymes), it could exploit energy flows to persist.
This is the heart of chemical evolution—molecules that are better at persisting and copying will naturally become more common over time, even in a universe trending toward greater entropy.
4. Is Life “Delaying” Entropy?
- Yes, in a local sense. Life organizes energy into complex structures, creating temporary order.
- No, in the universal sense. Life increases overall entropy by using energy, releasing heat, and eventually breaking down.
5. The Big Picture: Why Did Order Arise at All?
One possibility is that the laws of physics naturally allow complexity to emerge from chaos when energy flows through a system. This means:
- Entropy doesn’t mean pure randomness; it just means energy spreads out.
- Under the right conditions, complex molecules like RNA could emerge naturally.
Some scientists suggest that self-organizing systems—from hurricanes to galaxies to life—are a natural consequence of how the universe works.
So life is a symptom of entropy of its not resisting it?
Yes! Life can be seen as a symptom of entropy, rather than something that resists it. This perspective flips the common idea of life fighting against disorder and instead suggests that life is a natural consequence of entropy increasing in the universe.
1. Life as an Entropic Process
The second law of thermodynamics states that entropy (disorder) always increases in a closed system. However, life appears highly ordered, which seems contradictory—until you consider how life processes energy.
- Life does not violate the second law because it is not a closed system.
- Instead, it takes in high-energy, low-entropy inputs (like sunlight or chemical energy).
- Then it releases low-energy, high-entropy outputs (like heat and waste).
This means that life speeds up the process of entropy increase, just in a structured way.
Example: Earth and the Sun
- The Sun emits highly ordered low-entropy energy (visible light).
- Life on Earth absorbs this energy, organizes itself temporarily, and releases higher-entropy energy (heat).
- The net effect? Entropy still increases, but life emerges as a process along the way.
2. Why Life is Not “Resisting” Entropy
At first glance, life seems to resist entropy because it maintains structure and complexity. But:
- Life only maintains its order by increasing entropy elsewhere.
- A living system requires energy input—without it, disorder takes over (decay, death).
- When an organism dies, it decomposes—and its molecules spread out, increasing entropy.
Thus, life is a temporary, organized channel through which entropy increases more efficiently.
3. Is Life an Inevitable Result of Entropy?
Some scientists argue that life isn’t just compatible with entropy—it might actually be a necessary consequence of energy flowing through complex systems.
- Dissipative Structures: In physics, systems that take in energy and create temporary order (like hurricanes, galaxies, and life) are called dissipative structures.
- Life as an Entropy Catalyst: Life may have emerged because it was the most efficient way to break down energy gradients in the environment.
- Life on Other Planets? If life is a natural way to maximize entropy dissipation, then it might not be rare—it could be an expected consequence of energy-rich environments!
4. The Big Question: Is There a “Purpose” in Entropy Driving Life?
This is where science meets philosophy. Some perspectives to consider:
A. The Universe is Just Playing Out Physical Laws
- Life is simply one of many ways entropy increases.
- There is no “purpose,” just cause and effect at a massive scale.
B. Life is the Universe Understanding Itself
- Since entropy drives the emergence of complex systems, maybe intelligence is inevitable.
- Some suggest life, and eventually consciousness, is how the universe becomes self-aware.
- This ties into ideas in panpsychism, emergent complexity, and even metaphysics.
C. A Guided Process?
- Some see the fine-tuning of the universe (like the precise balance of physical constants) as evidence of a guiding force.
- If life is the most efficient way to increase entropy, is there a deeper reason why entropy needs to increase?
- This could be a scientific mystery or a philosophical/spiritual question.
5. Final Thought: Life as an Entropic “Flame”
Think of life like a candle flame:
- A flame doesn’t resist entropy—it’s a process of transformation, taking in fuel and oxygen and releasing heat and gases.
- Life works the same way—it’s an active process, temporarily organizing matter as it helps spread out energy.
Eventually, the candle burns out, just as individual organisms die, species go extinct, and even stars collapse. But while the flame is burning, it is part of the grand unfolding of entropy.
Gasp… pant… gasp… ok… gasp… sooo… pant…
LASTLY: (and the main question I want to ask you)
Is about dissipative structures because I’m seeing patterns her. Patterns in the universe. The universe dispersing energy more efficiently because of cause and effect. If we are to realize that everything fundamentally is made up of the same thing then it’s reasonable to assume it behaves similarly, ie dissipative structures, the hurricanes, the galaxies, and even living organisms, displaying channels to more efficiently disperse energy. Do they exist because entropy is always increasing? If so it’s all alike. Akin to each other in some way. The motion, the energy, the patterns, the spirals.
Spiral out, keep going, spiral out, keep going…